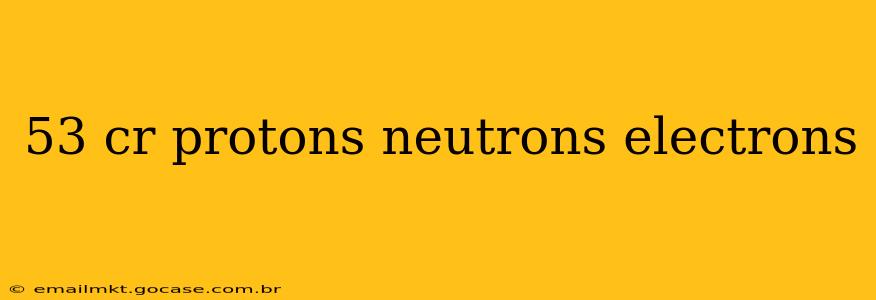
Understanding the Composition of Element 53: Iodine
Element 53, iodine (I), is a fascinating element with unique properties and crucial biological roles. Let's delve into its composition to understand its protons, neutrons, and electrons.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we look specifically at iodine, it's crucial to understand the basic building blocks of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; all iodine atoms have 53 protons.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. In a neutral atom, the number of electrons equals the number of protons.
Iodine's Composition: Protons, Neutrons, and Electrons
Now, let's apply this to iodine (I):
- Protons: As stated earlier, iodine always has 53 protons. This is what makes it iodine and distinguishes it from all other elements.
- Electrons: In a neutral iodine atom, there are 53 electrons to balance the positive charge of the 53 protons.
- Neutrons: This is where things get a bit more complex. Iodine has several isotopes, meaning atoms with the same number of protons but a different number of neutrons. The most common isotope, Iodine-127 (¹²⁷I), has 74 neutrons. Other isotopes exist, with varying neutron counts, but ¹²⁷I makes up the vast majority of naturally occurring iodine.
Frequently Asked Questions (Addressing potential "People Also Ask" queries):
What are the isotopes of iodine?
Iodine has several isotopes, the most stable and abundant being Iodine-127 (¹²⁷I). Other isotopes, such as Iodine-125 (¹²⁵I) and Iodine-129 (¹²⁹I), exist but are less common. These isotopes differ in their number of neutrons, influencing their stability and radioactivity. Some isotopes, like Iodine-131 (¹³¹I), are radioactive and used in medical applications, such as treating thyroid cancer, but they also pose health risks due to their radioactivity.
How many electron shells does iodine have?
Iodine has five electron shells. These shells are filled according to specific rules and principles of atomic structure, with the outermost shell containing the valence electrons which determine its chemical reactivity.
What is the atomic mass of iodine?
The atomic mass of iodine is approximately 126.90 atomic mass units (amu). This is a weighted average of the masses of all naturally occurring iodine isotopes, with ¹²⁷I contributing the most significantly to this average.
What is the chemical symbol for iodine?
The chemical symbol for iodine is I.
What is iodine used for?
Iodine is essential for human health, primarily in the production of thyroid hormones. It's also used in various industrial applications, including disinfectants and photographic chemicals. Iodine deficiency can lead to serious health problems, like goiter and hypothyroidism.
Conclusion:
Understanding the composition of iodine—its 53 protons, typically 74 neutrons (in its most common isotope), and 53 electrons—is key to understanding its chemical behavior and biological significance. This fundamental knowledge helps explain iodine's properties and its crucial role in various applications, from medicine to industry.